The Fundamentals of Mineral Base Oil Refining
Approximately 95 percent of the current lubricant market share is comprised of conventional (mineral-based) oils. Most people know these mineral oils are derived from a crude stock, but how much do you really know about the refining process?
The petroleum that flows from a well in the form of crude oil comes in many varieties and types, ranging from light-colored oils containing mostly small hydrocarbon molecular chains to black, nearly solid asphalt-like large hydrocarbon chains. These crude oils are very complex mixtures containing a plethora of different compounds made of hydrogen and carbon. These compounds (known as hydrocarbons) can range in size from methane (containing one carbon and four hydrogen atoms) to massive structures with 60 or more carbon atoms. This molecular size distribution can be used to our advantage.
The Importance of Refineries
Most lubricating oils come from petroleum or crude oil. In order to get a lubricating oil from a crude oil, the crude oil must be sent through a refinery. The refinery takes from the crude oil a lot of molecules of various sizes and structures that can be used for different things. For example, gasoline, diesel and kerosene are all derived from crude oil. Lubricating oil relates to hydrocarbon molecules of a particular size (in the range from 26 to 40 carbons). Fairly large and heavy molecules are needed to work as lubricating oils. The molecules that are used with gasoline and kerosene are smaller and have fewer carbons in the structure of the molecule. The refinery puts these molecules in little silos based on size and weight, and removes impurities, enabling each of the products from the crude oil to be utilized.
After the crude oil is desalted and sent through a furnace where it is heated and partially vaporized, it is sent to a fractionating column. This column operates slightly above atmospheric pressure and separates the hydrocarbons based on their boiling points, which are directly affected by their molecular size. In the fractionating column, heat is applied and concentrated at the bottom. The hydrocarbons entering the column will be vaporized. As they travel upward in the column, they will cool until they condense back into a liquid form. The point at which this condensation occurs varies again based in part on the molecular size.
By pulling the condensing liquid from the column at different heights, you can essentially separate the crude oil based on molecular size. The smallest of the hydrocarbons (5 to 10 carbon atoms) will rise to the very top of the column. They will be processed into products like gasoline. Condensing just before reaching the top, the compounds containing 11 to 13 carbon atoms will be processed into kerosene and jet fuel. Larger still at 14 to 25 carbon atoms in the molecular chain, diesel and gas oils are pulled out.
Those compounds with 26 to 40 carbon atoms are a tribologist’s main concern. This is the material used for the creation of lubricating oil. At the bottom of the column, the heaviest and largest of the hydrocarbons (40-plus carbon atoms) are taken and used in asphaltic-based products.
After the distillation process, the compounds need to be refined for their intended purpose. This step in the process is done to reduce the tendency of the base oil to age (oxidize) in service and also to improve the viscosity/temperature characteristics. There are two ways this can be done. The first involves a separation process where there are two products being made: a desired lube product and undesirable byproducts. The second way, which is quickly becoming the favored of the two, is a conversion process. This process involves converting undesirable molecular structures into desirable structures with the use of hydrogen, heat and pressure.
Extraction Process
The following is a simplified description of the extraction process:
Deasphalting
Propane deasphalting takes the residuum from the very bottom of the column (the heaviest, largest molecules) and separates them into two products: tar and compounds that are similar to the lube distillates but have a higher boiling point. This material is called deasphalted oil, and it will be refined in the same manner as the lube distillates.
Solvent Extraction
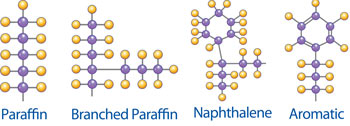
Solvent extraction is the term used for the removal of most of the aromatics and undesirable constituents of oil distillates by liquid extraction. Commonly used solvents contain phenol, furfural and sulphur dioxide. The resulting base stocks are raffinates (referred to as neutral oils) and an extract that is rich in aromatic content, which is highly sought after as a process oil or fuel oil.
Dewaxing
After solvent extraction, the raffinates are dewaxed to improve low-temperature fluidity. This process again produces two products: a byproduct wax that is almost completely paraffinic and a dewaxed oil that contains paraffins, naphthenes and some aromatics. This dewaxed oil becomes the base stock for many lubricants, but there is one more process that can be done to make a premium product.
Hydrofinishing
Hydrofinishing changes the polar compounds in the oil by a chemical reaction involving hydrogen. After this process, an observer would notice a lighter-colored product and an improved chemical stability. The final quality of the base oil is determined by the severity of the application of temperature and pressure in the hydrofining process.
Conversion Process
The following is a simplified description of the conversion process:
Hydrocracking
In this refining process, the distillates are subjected to a chemical reaction with hydrogen in the presence of a catalyst at high temperatures and pressures (420 degrees C and 3,000 psi). The aromatic and naphthene rings are broken, opened and joined using hydrogen to form an isoparaffin structure. The reaction with hydrogen will also aid in the removal of water, ammonia and hydrogen sulfide.
Hydrodewaxing
During hydrodewaxing, much like hydrocracking, a hydrogenation unit is used to deploy a catalyst that is specific to conveying waxy normal paraffins to more desirable isoparaffin structures.
Hydrotreating
| Charachteristic | Difference |
|---|---|
| Color | Conversion produces clear, colorless products |
| Viscosity Index | Conversion produces higher viscosity indexes |
| Oxidation Resistance | Conversion produces better oxidation resistance |
| Thermal Stability | Disappears when power supply is shut off |
| Carbon Residue | Conversion products produce fewer residuals |
| Demulsibility | Conversion products separate water more readily |
| Biodegradability | Conversion products tend to biodegrade faster |
Because the previous two processes involve breaking chemical bonds between two carbon atoms, it is necessary to introduce the saturation of any unsaturated molecules. This is easily done by introducing more hydrogen. These saturated molecules are more stable and will be able to resist the oxidation process better than the unsaturated variety.
There are slight differences in the characteristics of the finished base oil produced by these two processes. The main difference lies in the aromatic content. The conversion process can reduce the aromatic content to around 0.5 percent, while the extraction process lingers around 15 to 20 percent. This aromatic content has the following effects:
It would appear that the conversion process produces a better quality product, but there is always a trade-off. The cost of refining oil using the conversion process is somewhat higher than the extraction process. This extra cost incurred by the refiner is eventually passed on to the customer. However, in this case, the customer typically gets what he pays for - a higher quality base oil at a higher initial price.
