Synthetic Esters: Engineered to Perform
By Tyler Housel, Inolex Chemical Co.
The lubricant industry generally treats synthetic esters as a monolithic class of Group V base oils with well-defined properties. It is not difficult to find a chart that lists esters as having “fair” hydrolytic stability, “good” biodegradability, “very good” lubricity, “excellent” oxidative stability and so on. Sometimes diesters and polyol esters are listed separately, but there is seldom further differentiation. However, the nature of esters defies such oversimplification. There are endless varieties of esters that can be built from commonly available acids and alcohols, so almost anything is possible.
Modern synthetic esters can be “tuned” to perform in nearly any environment and application. Whether you seek excellent hydrolytic stability, oxidative stability, biodegradability, lubricity, high viscosity index or low-temperature properties, all of these are possible with the right synthetic ester. Synthetic esters are manufactured from carboxylic acids and alcohols, which are very common chemical building blocks. They provide almost unlimited structural and performance possibilities.
The Ester Reaction
Figure 1 shows the basic chemical reaction used to synthesize all esters - a carboxylic acid and an alcohol react to form an ester and water. Organic chemists call this a reversible reaction because water can react with ester groups and break the ester into its components. This is known as hydrolysis.
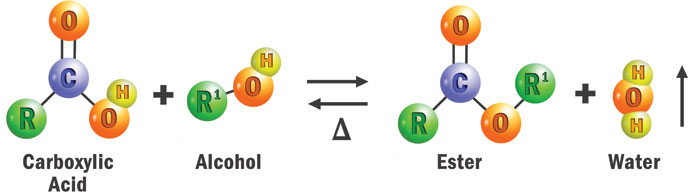
The raw materials used to make esters can be linear, branched, saturated, unsaturated, monofunctional, difunctional or polyfunctional. There are hundreds of potential acid and alcohol building blocks, and the number of combinations is almost limitless. Attempts have been made to classify esters in categories such as diesters and polyol esters or simple and complex esters, but the technology is far ahead of the terminology.
The building blocks often define the maximum performance potential of an ester, while the manufacturing savvy determines whether the ester reaches its potential. For example, a synthetic neopolyol (alcohol) can produce an ester with outstanding oxidative stability, yet the oxidative stability of the ester may be diminished with inferior ingredients, contaminants or poor processing techniques.
Thermo-oxidative Stability
Oxidation is a degradation process that occurs when atmospheric oxygen reacts with organic molecules. For synthetic esters, this normally occurs at high temperatures, but it is possible to find esters that oxidize without heating. It has been known for centuries that linseed oils form a solid coating when exposed to air at ambient temperatures. These are called drying oils because they can be painted on wood and cured to a hard, protective varnish. Room-temperature polymerization relies on oxidative cross-linking of polyunsaturated fatty acids.
While varnish enhances the appearance of antique furniture, it is not beneficial on industrial equipment. Synthetic esters are the best choice to provide clean, varnish-free lubrication at temperatures up to 600 degrees F (300 degrees C). The only way to engineer a superior high-temperature lubricant is to understand and eliminate structures that are oxidatively unstable.
It has already been established that polyunsaturated fatty acid components must be eliminated, but unsaturated fatty acids such as oleates are commonly used in lubricants. In fact, oleates have many good properties including lubricity, low volatility, cold flow, biodegradability, renewability and a low price. The oxidative stability is also much better than that of drying oils. However, unsaturated esters, including vegetable oils, are still limited to lower temperature applications.
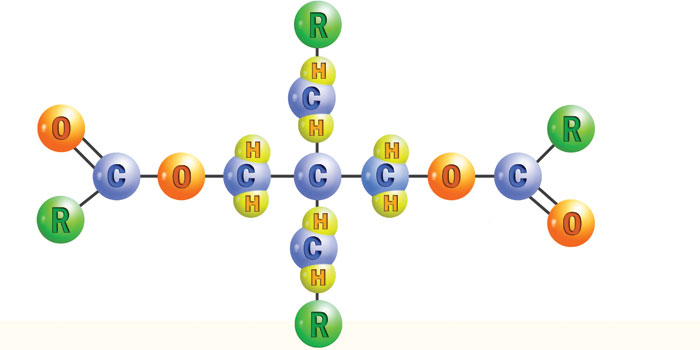 Saturated esters are required for use at higher temperatures, but there is more to consider. High-temperature oxidative stability depends heavily on the amount and configuration of hydrogen on the beta-carbons in the molecule. The beta-carbon is the second one from the carbon-oxygen bond of the ester group. The beta-hydrogen is very reactive toward oxygen, so esters with no beta-hydrogen are more thermally stable. These are known as neopolyol esters, with their name derived from their structural similarity to neopentane. Neopolyol is shortened to polyol esters and abbreviated as POE. All POEs have good oxidative stability because they have no beta-hydrogens (see Figure 2).
Saturated esters are required for use at higher temperatures, but there is more to consider. High-temperature oxidative stability depends heavily on the amount and configuration of hydrogen on the beta-carbons in the molecule. The beta-carbon is the second one from the carbon-oxygen bond of the ester group. The beta-hydrogen is very reactive toward oxygen, so esters with no beta-hydrogen are more thermally stable. These are known as neopolyol esters, with their name derived from their structural similarity to neopentane. Neopolyol is shortened to polyol esters and abbreviated as POE. All POEs have good oxidative stability because they have no beta-hydrogens (see Figure 2).
Although unsaturated fatty acids cannot perform at high temperatures, it is not enough to simply substitute saturated fatty acids such as stearic acid. Synthetic short-chain carboxylic acids offer a greater degree of oxidative stability and are much better at low temperatures than saturated fatty acids. Shorter branched fatty acids are used when exceptional thermal stability is required. By eliminating the oxidative weak points, synthetic esters can be designed to operate at high temperatures and will tend to evaporate cleanly before undergoing oxidative polymerization so they will not form deposits and varnish.
Viscosity
Chemists find many examples of the link between viscosity and molecular weight. From linear alkanes to polymers, bigger molecules are expected to be more viscous. However, this simple rule of thumb does not always apply to synthetic esters. The viscosity is strongly dependent on the amount of branching, aromaticity, functionality and ease of rotation of the bonds that make up the molecule. As the structure becomes more branched, it is more difficult for the molecule to bend around and flow over itself.
Aromatic esters are extremely viscous because of the rigid aromatic ring. So while it’s true that molecular weight is related to viscosity, there are also ways to break this relationship when desired. This is particularly useful when the volatility profile requires a specific molecular weight and the application demands a certain viscosity.
Molecular weight is not the only factor that determines the viscosity of a synthetic ester, but it can certainly be used to increase viscosity when necessary. If the component acids and alcohols each have more than one reactive group, esters can be polymerized to any length. Although the lubricant industry doesn’t employ rigid polyesters that are made into bottles, the same principle can be used to build molecular weight and therefore increase viscosity. These are called complex esters or CPE.
Biodegradability and Hydrolytic Stability
The rate of the hydrolysis reaction is highly dependent on both the chemistry of the ester bond and the environmental conditions. Synthetic esters can be stable for a few hours or thousands of years, so it is impossible to classify them using words such as “fair” or “good.” To manage hydrolysis, it is important to understand the type and purity of the reactants as well as the manufacturing process.
Remember that esters are made from alcohols and carboxylic acids, and that water is a byproduct of the esterification reaction. All ester reactions are reversible, so water can break ester back into the acid and alcohol components. Once ester is broken into the alcohols and acids, bacteria can complete the digestion of the components. Typically, increasing the amount of natural components such as vegetable-based fatty acids helps biodegradability. When synthetic acids and neopolyol alcohols are used, the ester becomes more inert and the rate of biodegradation is reduced.
It is possible to chemically block the hydrolysis pathway using branched carboxylic acids. These esters are extremely stable in water and act like mineral oils in typical hydrolysis tests. In fact, a computer simulation shows that the rate of hydrolytic degradation is measured in hundreds of years.
Smoke Point, Flash Point, Fire Point and Volatility
| Synthetic Base Oil | Viscosity at 40°C | Flash Point |
|---|---|---|
| PAO | 19 cSt | 220°C |
| PAG | 34 cSt | 218°C |
| Alkylated Naphthalene | 29 cSt | 222°C |
| Diester | 14 cSt | 231°C |
| Polyolester (POE) | 19 cSt | 257°C |
Synthetic esters are prized for their ability to lubricate at high temperatures. One of the main reasons for this is that they have a much lower volatility than other lubricant base oils at a given viscosity. Volatility is strongly related to smoke point, flash point and fire point, which are part of ASTM D-92. As the temperature rises, the amount of evaporation increases until there is visible smoke and eventually enough smoke to support a flash or fire in the presence of a flame. The table on page 40 shows the relationship between flash point and viscosity for several common types of synthetic lubricants.
Volatility is also dependent on the distribution of molecular weight in a lubricant. It has been proven that a small amount of flammable solvent will still be flammable even if mixed with other inert components. The mixture will ignite as long as there is enough flammable vapor in the air. Likewise, the most volatile components of a lubricant base oil determine flash point. Esters can be designed to have a very pure composition so there are few small molecules to smoke and flash. An added benefit is that viscosity stays in grade because no light ends evaporate from the lubricant.
Volatility and Deposits
From a chemical standpoint, volatility is related to molecular weight, polarity and chemical stability. While molecular weight and polarity are well-known effects, chemical stability is often overlooked because it considers only small organic molecules. However, a high-temperature lubricant is made from larger molecules that do not evaporate readily, so stability becomes important.
Oxidative and thermal degradation begin to occur between 200 to 300 degrees C. At these temperatures, base oil evaporation is a slow process. However, oxidation can break the molecule into small, volatile fractions. A large percentage of the weight loss in evaporation tests such as ASTM D-2595 comes from oxidation. Not only does oxidation cause weight loss, but it also causes varnish. The decomposition products in the vapor phase are often free radicals or reactive molecules. Deposits and varnish can form as the radical groups in the vapor condense and create a polymer varnish on metal surfaces. These polymers can also form sludge if they reach a high enough concentration to be insoluble in the bulk oil.
Synthetic esters reduce varnish and other deposits because they have outstanding oxidative stability and do not form many radical decomposition products. Furthermore, they are good high-temperature solvents and tend to dissolve the varnish back into the liquid phase so it can be filtered out.
Lubricity, Polarity and Additives
The key property of a lubricant is that it is expected to lubricate. Lubricity has to do with how easily the molecule flows over itself and how well it competes for and coats the metal surface. Esters are generally considered good boundary lubricants because they associate with metal surfaces and reduce the amount of metal-to-metal contact during sliding motion. Structural factors that impact lubricity include the chain length, the amount of branching and the location of linkages within the molecule.
Longer carbon chains, less branching and good polarity all favor boundary lubrication. Ester linkages are polar but can be less surface active if they are shielded by carbon chains. Synthetic esters are designed from different acid and alcohol feed stocks, so the location of ester groups and type of carbon chains can be selected independently. The lubricity of the ester base stock depends on the interaction of the ester with the metal surface. Esters have good lubricity, but under severe conditions, anti-wear and extreme-pressure additives are used to carry the bulk of the load. Some say esters compete so vigorously for the metal surfaces that they crowd out necessary additives. However, many additives are active enough to displace an ester from a surface. Expertise and experience are important here, as some additives do not work well with synthetic esters.
It is also important to choose an ester that is appropriate for the application. If the application involves boundary lubrication where metal surfaces grind together under pressure, lubricity is a key concern. But if the application involves only hydrodynamic lubrication where there’s no metal-to-metal contact, lubricity is less important. Esters are great for high-temperature hydrodynamic applications because they can survive in extreme environments where no other lubricant can.
Manufacturing, Chemical Stability and Application Suitability
To this point, the role that chemical structure plays in ester properties has been discussed. However, a second factor is equally important: the manufacturing process and the residuals it can leave behind.
Residual Acid Value
Ester manufacturing always starts with an acid and an alcohol, both of which may be volatile. It is impossible to achieve 100-percent conversion in any chemical reaction, so there is always some residual carboxylic acid or alcohol in the final product. If this is not properly controlled, it can alter the initial properties of the ester and can also cause the lubricant’s properties to change during storage and use.
Carboxylic acids are the primary concern because they can accelerate hydrolytic breakdown of the lubricant. This is especially problematic in metalworking fluids where water is a main component.
 Figure 3 shows the effect of residual acid on hydrolytic stability. This is an accelerated hydrolysis test that holds an ester and water in a sealed tube at 125 degrees C. Ester 1 (blue) has an acid number of 0.03 milligrams of potassium hydroxide per gram (mg KOH/g) and shows almost no degradation over the duration of the test. Ester 2 (purple) starts with an acid number of 1, while Ester 3 (red) begins with an acid number of 3. Esters 2 and 3 are highly degraded by the end of the test.
Figure 3 shows the effect of residual acid on hydrolytic stability. This is an accelerated hydrolysis test that holds an ester and water in a sealed tube at 125 degrees C. Ester 1 (blue) has an acid number of 0.03 milligrams of potassium hydroxide per gram (mg KOH/g) and shows almost no degradation over the duration of the test. Ester 2 (purple) starts with an acid number of 1, while Ester 3 (red) begins with an acid number of 3. Esters 2 and 3 are highly degraded by the end of the test.
Catalyst Residue
Esters are usually made with a catalyst to accelerate the synthesis, but ester catalysts also accelerate the degradation of ester in the presence of water. Therefore, it is essential to remove or deactivate the ester catalyst at the end of the manufacturing process to ensure that the ester will maintain its quality during storage, formulation and use.
Moreover, mineral acids and certain active metals must be avoided because they can break down any type of ester. Most ester lubricants are not recommended for applications in which they will come in contact with strong acids and bases.
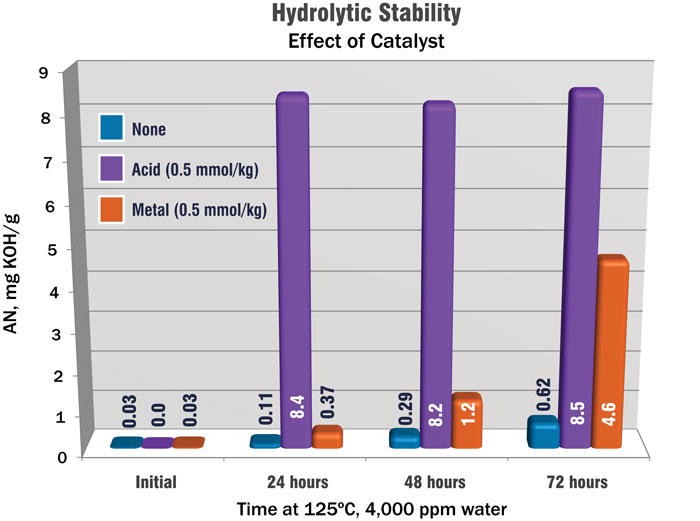 Figure 4 shows the effect of mineral acids and metals on hydrolytic stability. The three samples all started with virtually no acid present. One sample (purple) was treated with a mineral acid, while metal fines were added to another (red). As shown on the left, the strong mineral acid completely hydrolyzed the sample within 24 hours. Metal fines were not as fast but had the same effect. The untreated sample (blue) retained its integrity.
Figure 4 shows the effect of mineral acids and metals on hydrolytic stability. The three samples all started with virtually no acid present. One sample (purple) was treated with a mineral acid, while metal fines were added to another (red). As shown on the left, the strong mineral acid completely hydrolyzed the sample within 24 hours. Metal fines were not as fast but had the same effect. The untreated sample (blue) retained its integrity.
In conclusion, it is a good idea to consider the expertise and experience of your ester supplier. Esters can be designed and manufactured to work in almost any environment, but this means the selection process is critical. Work with someone who knows the science and technology of esters and is willing to take the time to understand your requirements. This is the only way to ensure you are getting the right product for your lubrication needs.
