Understanding the Differences Between Base Oil Formulations
All lubricants contain a base oil. It serves as the foundation of the lubricant before it is blended with additives or a thickener in the case of a grease. But how do you know which base oil is best? Trying to choose between mineral oils and synthetics can be confusing. This article will break down the complexity between base oil formulations so you can make the right decision for each application.
Base Oil Categories
Lubricants can be categorized in many different ways. One of the most common classifications is by the constituent base oil: mineral, synthetic or vegetable. Mineral oil, which is derived from crude oil, can be produced to a range of qualities associated with the oil’s refining process. Synthetics are man-made through a synthesizing process and come in a number of formulations with unique properties for their intended purpose. Vegetable base oils, which are derived from plant oils, represent a very small percentage of lubricants and are used primarily for renewable and environmental interests.
55% of lubrication professionals use both synthetic and mineral-based lubricants at their plant, according to a recent survey at MachineryLubrication.com
 Base Oil Characteristics
Base Oil Characteristics
All base oils have characteristics that determine how they will hold up against a variety of lubrication challenges. For a mineral oil, the goal of the refining process is to optimize the resulting properties to produce a superior lubricant. For synthetically generated oils, the objective of the various formulations is to create a lubricant with properties that may not be achievable in a mineral oil. Whether mineral-based or synthetic-based, each base oil is designed to have a specific application.
Some of the most important base oil properties include the viscosity limitations and viscosity index, pour point, volatility, oxidation and thermal stability, aniline point (a measure of the base oil’s solvency toward other materials including additives), and hydrolytic stability (the lubricant’s resistance to chemical decomposition in the presence of water).
| Synthetic | Strengths | Weaknesses |
|---|---|---|
| Polyalphaolefins (PAOs) Maximum Operating Temperature: 270°F/132°C |
High VI, high thermal oxidative stability, low volatility, good flow properties at low temperatures, nontoxic and compatible with mineral oils | Limited biodegradability, limited additive solubility, seal shrinkage risk |
| Diesters and Polyolesters Maximum Operating Temperature: 360°F/182°C |
Nontoxic, biodegradable, high VI, good low-temperature properties, miscible with mineral oils | Low viscosities only, bad hydrolytic stability, limited seal and paint compatibility |
| Phosphate Esters Maximum Operating Temperature: 240°F/116°C |
Fire resistant, biodegrades quickly, excellent wear resistance and scuffing protection | Low VI, limited seal compatibility, not miscible with mineral oils, moderate hydrolytic stability |
| Polyalkylene Glycols (PAGs) Maximum Operating Temperature: 300°F/149°C |
Excellent lubricity, nontoxic, good thermal and oxidative stability, high VI | Additives marginally miscible, not miscible with mineral oils, limited seal and paint compatibility |
| Silicones Maximum Operating Temperature: 450°F/232°C |
Highest VI, high chemical stability, excellent seal compatibility, very good thermal and oxidative stability | Worst mixed and boundary film lubrication properties, not miscible with mineral oils or additives |
 Base Oil Groups
Base Oil Groups
The 20th century saw a number of improvements in the refining process used for mineral oils along with the introduction of a variety of synthetics. By the early 1990s, the American Petroleum Institute (API) had categorized all base oils into five groups, with the first three groups dedicated to mineral oils and the remaining two groups predominantly synthetic base oils.
Groups I, II and III are all mineral oils with an increasing severity of the refining process. Group I base oils are created using the solvent-extraction or solvent-refining technology. This technology, which has been employed since the early days of mineral oil refining, aims to extract the undesirable components within the oil such as ring structures and aromatics.
Group II base oils are produced using hydrogen gas in a process called hydrogenation or hydrotreating. The goal of this process is the same as for solvent-refining, but it is more effective in converting undesirable components like aromatics into desirable hydrocarbon structures.
Group III base oils are made in much the same way as Group II mineral oils, except the hydrogenation process is coupled with high temperatures and high pressures. As a result, nearly all undesirable components within the oil are converted into desirable hydrocarbon structures.
When comparing properties among the mineral base oil groups, you typically will see greater benefits with those that are more highly refined, including those with enhanced oxidation stability, thermal stability, viscosity index, pour point and higher operating temperatures. Of course, as the oil becomes more refined, some key weaknesses also occur, which can affect additive solubility and biodegradability.
Group IV is dedicated to a single type of synthetic called polyalphaolefin (PAO). It is the most widely used synthetic base oil. PAOs are synthetically generated hydrocarbons with an olefinic tail formed through a polymerization process involving ethylene gas. The result is a structure that looks very much like the purest form of the mineral oils described in Group III. The advantages of PAOs over mineral oil include a higher viscosity index, excellent low- and high-temperature performance, superior oxidation stability, and lower volatility. However, these synthetic lubricants can also have deficiencies when it comes to additive solubility, lubricity, seal shrinkage and film strength. Much like mineral oils, PAOs are widely employed for lubricating applications and are often the preferred option when higher temperatures are expected.
Group V is assigned to all other base oils, particularly synthetics. Some of the most common oils in this group include diesters, polyolesters, polyalkylene glycols, phosphate esters and silicones.
Diester (dibasic acid ester) is manufactured through a reaction of dibasic acid with alcohol. The resulting properties can be adjusted based on the types of dibasic acid and alcohol used.
Polyolester is made through a reaction of monobasic acid with a polyhydric alcohol. Much like diesters, the resulting properties will depend on these two constituent types.
Polyalkylene glycol (PAG) is produced through a reaction involving ethylene or propylene oxides and alcohol to form various polymers. A number of PAG products are developed based on the oxide used, which will ultimately influence the base oil’s water solubility.
Phosphate ester is created through a reaction of phosphoric acid and alcohol, while silicones are formulated to have a silicon-oxygen structure with organic chains attached. Each of these synthetics has specific strengths and weaknesses, as shown in the table above.
Applications
In general, synthetics can provide greater benefits when it comes to properties influenced by extreme temperatures, such as oxidative and thermal stability, which can contribute to an extended service life. In situations where the lubricant will encounter cold startups or high operating temperatures, synthetics like PAOs typically will perform better than mineral oils. PAOs also exhibit improved characteristics in relation to demulsibility and hydrolytic stability, which influence the lubricant’s ability to handle water contamination.
While PAOs are ideal for applications like engine oils, gear oils, bearing oils and other applications, mineral oil remains the predominant oil of choice due to its lower cost and reasonable service capabilities. With more than 90 percent usage in the industrial and automotive markets, mineral oil has solidified its place as the most common base oil in the majority of applications.
4 Things to Know About Base Oils
- All oils from each base oil type are not the same, as the formulations can produce unique distinctions. Therefore, the properties described for each base oil type are generalized for the category as a whole.
- Group III oils are sometimes advertised as synthetics. There is an understanding that the refining process has severely modified the original hydrocarbon, thus synthesizing the more highly pure product.
- Water-based fluids are an alternative when fire resistance is imperative and typical lubricant properties like viscosity or lubricity are less important.
- Be cautious when switching lubricants, particularly when they have different base oils, as they may be incompatible with each other.
Paraffinic mineral oil, which is represented in Groups I, II and III, can offer a higher viscosity index and a higher flash point in comparison to naphthenic mineral oils, which have lower pour points and better additive solvency. Even though naphthenic oil is mineral-based, it is considered a Group V oil because it does not satisfy the API’s qualifications for Group I, II and III. The unique characteristics of naphthenic mineral oils have often made them good lubricants for locomotive engine oils, refrigerant oils, compressor oils, transformer oils and process oils. Nevertheless, paraffinic oils continue to be the preferred option for high-temperature applications and when longer lubricant life is required.
Ester-based synthetics, such as diesters and polyolesters, have advantages when it comes to biodegradability and miscibility with other oils. In fact, it is common for diesters and polyolesters to be mixed with PAOs during additive blending to help accept more significant additive packages. Diesters and polyolesters are often deployed as the base oil for compressor fluids, high-temperature grease applications and even bearing or gear oils. Because they are known to perform well at higher temperatures, polyolesters have also been widely used for jet engine oils.
Compared to other oils, polyalkylene glycols (PAGs) have a much higher viscosity index and good detergency, lubricity, and oxidative and thermal stability characteristics. PAGs can be formulated to be water soluble or insoluble and do not form deposits or residue during extreme operating conditions. PAGs can be employed in a number of applications, such as compressor oil, brake fluid, high-temperature chain oil, worm gear oil and metalworking fluid, as well as for applications with food-grade, biodegradability or fire-resistant requirements.
Phosphate esters are primarily beneficial for fire-resistant applications. They are often utilized in hydraulic turbines and compressors due to their unique properties, including high ignition temperatures, oxidation stability and low vapor pressures.
Silicone-based synthetics are infrequently used in industrial applications, but they can be advantageous in extremely high temperatures, when the lubricant will contact chemicals, or when exposed to radiation or oxygen. These synthetics have a very high viscosity index and are among the best options for oxidation and thermal stability because they are chemically inert.
Selecting a Base Oil
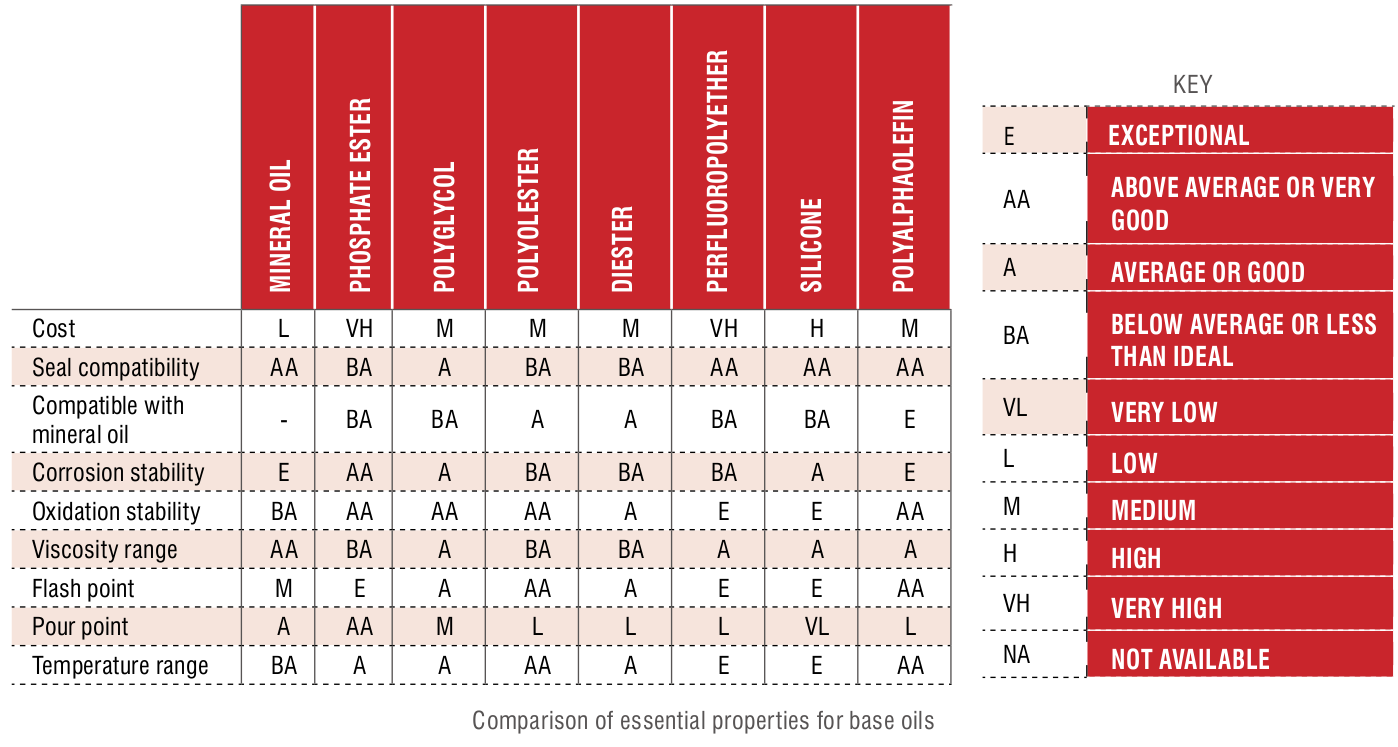
When you are choosing a base oil, there will be trade-offs in the lubricant properties required for the application. A common example is viscosity. Higher viscosity provides adequate film strength, while lower viscosity offers low-temperature fluidity and lower energy consumption. In some cases, you may prefer to have a balance between the two so there isn’t too much of a compromise on either side. The chart on page 33 shows a comparison of the most essential properties for each base oil.
Although it’s not necessarily important to understand the way in which the oil was manufactured, it is critical to know the available base oil options and the advantages and disadvantages they provide. Optimizing your lubricant selection can help minimize the opportunities for machine failure. While synthetics are justifiably more expensive than mineral oil, the cost of equipment failure is typically much higher. If cost is a key factor in your decision, be sure to choose wisely.
References
Wright, J. (2009). “Phosphate Ester Fluids - Benefits and Limitations.” Machinery Lubrication, Vol. 9, Issue 6.
Greaves, M.R. (2013). “Polyalkylene Glycols: Present and Future Applications.” Webinar.
Jackson, A. (1987). “Synthetic versus Mineral Fluids in Lubrication.” Keynote Address to International Tribology Conference. Melbourne, Australia.
Cash, W. (2015). “Understanding the Differences Between Synthetics.” Machinery Lubrication, Vol. 15, Issue 3.
Fitch, B. (2013). “When Are Mineral Oils Superior to Synthetics?” Reliable Plant Conference Proceedings.
Fitch, J.C., Scott, R., & Leugner, L. (2012). “The Practical Handbook of Machinery Lubrication - Fourth Edition.”
